Bedroom carbon dioxide buildup
By Michael Gebis, Mon 16 November 2020, in category Citizen-science
By Michael Gebis, Mon 16 November 2020, in category Citizen-science
You probably already know that carbon monoxide (CO) from combustion is highly poisonous. Hemoglobin binds to CO tighter than it binds to O2 by a factor of 200, so a small amount quickly prevents your blood cells from being able to carry oxygen, and you die. That's why you need to have a CO detector in your house--you need to catch it quick.
But what about carbon dioxide (CO2)--you know, the stuff you breathe out? It's usually considered relatively inert, and until recently, I thought it was nearly inert and basically harmless, as long as you were still getting oxygen. But it turns out that CO2 can cause ill effects at levels starting around 1000ppm.
A few years ago, I bought an "AirVisual Pro Smart Air Quality Monitor" to monitor indoor particulate matter. I had it in my front room, and was able to optimize my air filters and get my PM 2.5 under control. It also monitors CO2 levels, which I really didn't care about at the time.
But I recently moved the monitor into my bedroom. And now that it's getting colder, I started sleeping with my window closed. Bad idea.
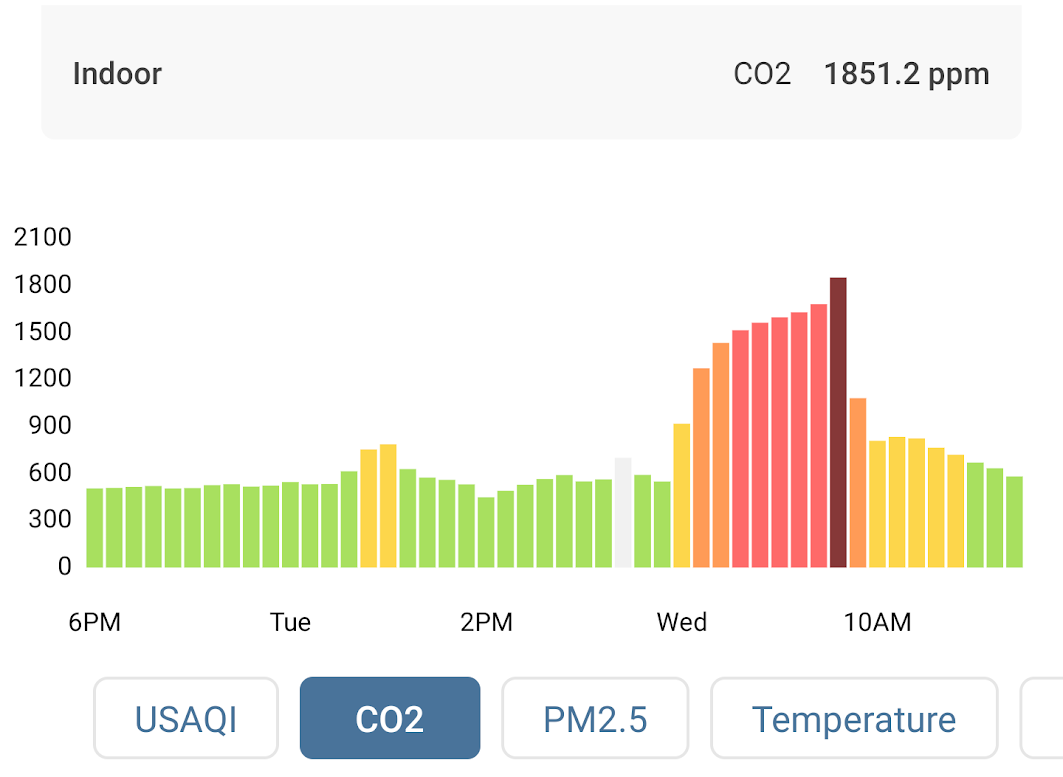
On Wednesday, the night I closed my window, the CO2 in my bedroom reached as high as 1850 ppm, which is enough to cause drowsiness and headaches. I am sure I was getting terrible sleep. I was shocked!
| CO2 concentration | Effect |
|---|---|
| 400 ppm | the "normal level" of atmospheric CO2 in 2020. |
| 1000-2000 ppm | drowsiness, "stale air" |
| 2000-5000 ppm | headaches, sleepiness, poor concentration, slight nausea, increased heart rate |
| 5000 ppm+ | workplace exposure limit |
Needless to say, I have since slightly cracked my window, which is enough to keep CO2 levels below 700 ppm at their worst. Still not great, but a reasonable compromise between temperature and CO2 levels.
CO2 is also dangerous when it displaces oxygen--CO2 is heavy, and if you generate a bunch of it, it can settle in a basin or poorly ventilated area, and just "push" all the oxygen out of the area.
CO2 buildup happens when dry ice melts, and every year there are multiple suffocations from melting dry ice. CO2 is also a byproduct of beer and bread fermentation, although it's unlikely you would be able to build up CO2 to fatal levels at home, it could happen in an industrial setting.
But in researching this article, I came across references to low-lying Lake Nyos in Cameroon. The lake slowly builds up CO2 and when it builds up to high enough levels, the lake burps it out all at once. And when this happens in 1986 more than 1700 people suffocated. Damn, nature, you scary! Fortunately there is now an automatic degassing system in place to prevent CO2 from building up under the lake, so hopefully this never happens again.